Nuclear receptors are the class of Intracellular receptors that are always present within the cell and similar to other receptors, they are also protein-base structures. These receptors get activated by lipid-soluble signals like estrogen, thyroid hormone, etc. that can cross the plasma membrane. Upon activation by these ligands, nuclear receptors bind to specific DNA sequences, regulating gene expression and consequently influencing various biological processes, including development, metabolism, reproduction, immune functions, etc.
All the nuclear receptors function as transcription factors and switch on or off specific genes based on incoming signals which means that upon binding with a signal, these receptors selectively regulate the genes that are associated with that particular signal, thus maintaining precise control over gene expression.
When it comes to cell surface receptors, they do not directly enter the nucleus to initiate DNA expression. Instead, they indirectly activate other molecules that subsequently enter the nucleus for gene expression. On the other hand, nuclear receptors bind directly to DNA and initiate transcription. The specific site where a nuclear receptor binds to DNA is referred to as the Hormone Response Element (HRE).
Nuclear receptors are known for their comparatively slower actions, distinct from the rapid responses seen in ligand-gated ion channels and G protein-coupled receptors (GPCRs). Despite their gradual nature, these actions are notably prominent. It’s noteworthy that the human genome encodes 48 known nuclear receptors.
TYPES OF NUCLEAR RECEPTORS
Nuclear receptors are classified into two primary categories: Type 1 and Type 2.
Type 1 nuclear receptors
Type 1 nuclear receptors reside in the cytoplasm initially and move into the nucleus upon binding to a ligand, where they activate gene expression.
Examples within the Type 1 nuclear receptor group are the Androgen Receptor (AR), Glucocorticoid Receptor (GR), Estrogen Receptor (ER), Progesterone Receptor (PR), Mineralocorticoid Receptor (MR), etc.
Type 2 Nuclear Receptors
On the other hand, Type 2 Nuclear receptors reside within the nucleus and remain anchored to specific DNA segment, regardless of ligand presence. Binding with a ligand triggers gene expression.
Examples within the Type 2 nuclear receptor category include the Retinoic Acid Receptor (RAR), Vitamin D Receptor (VDR), Peroxisome Proliferator-Activated Receptor (PPAR), and Liver X Receptor (LXR).
NATURE OF LIGAND FOR NUCLEAR RECEPTORS
Nuclear receptors are specialized receptors designed to respond to hydrophobic signals, which are essentially non-polar in nature. These ligands must be small in size and lipophilic to cross cell membranes. In contrast to surface receptors, such as GPCRs or Ion channel receptors, where ligands stay external, nuclear receptors require ligands to enter the cell. The plasma membrane, being hydrophobic itself, facilitates the entry of such signals into the cell. After the hydrophobic signal enters the cell, what happens next depends on the type of signal—whether it binds with the nuclear receptor found in the cytoplasm or the one situated within the nucleus. When developing drugs targeting nuclear receptors, they must be small and lipophilic to function effectively.
STRUCTURE OF NUCLEAR RECEPTORS
Nuclear receptors are highly conserved and very similar in structure in comparison with each other. They have a characteristic structure compartmentalized into distinct regions or domains, referred to as A/B, C, D, E, and F, with each domain serving a particular role in the receptor’s function and organization.
Nuclear receptors exist in a folded state, but let’s simplify their structure in a linear form and discuss each of these domains individually.
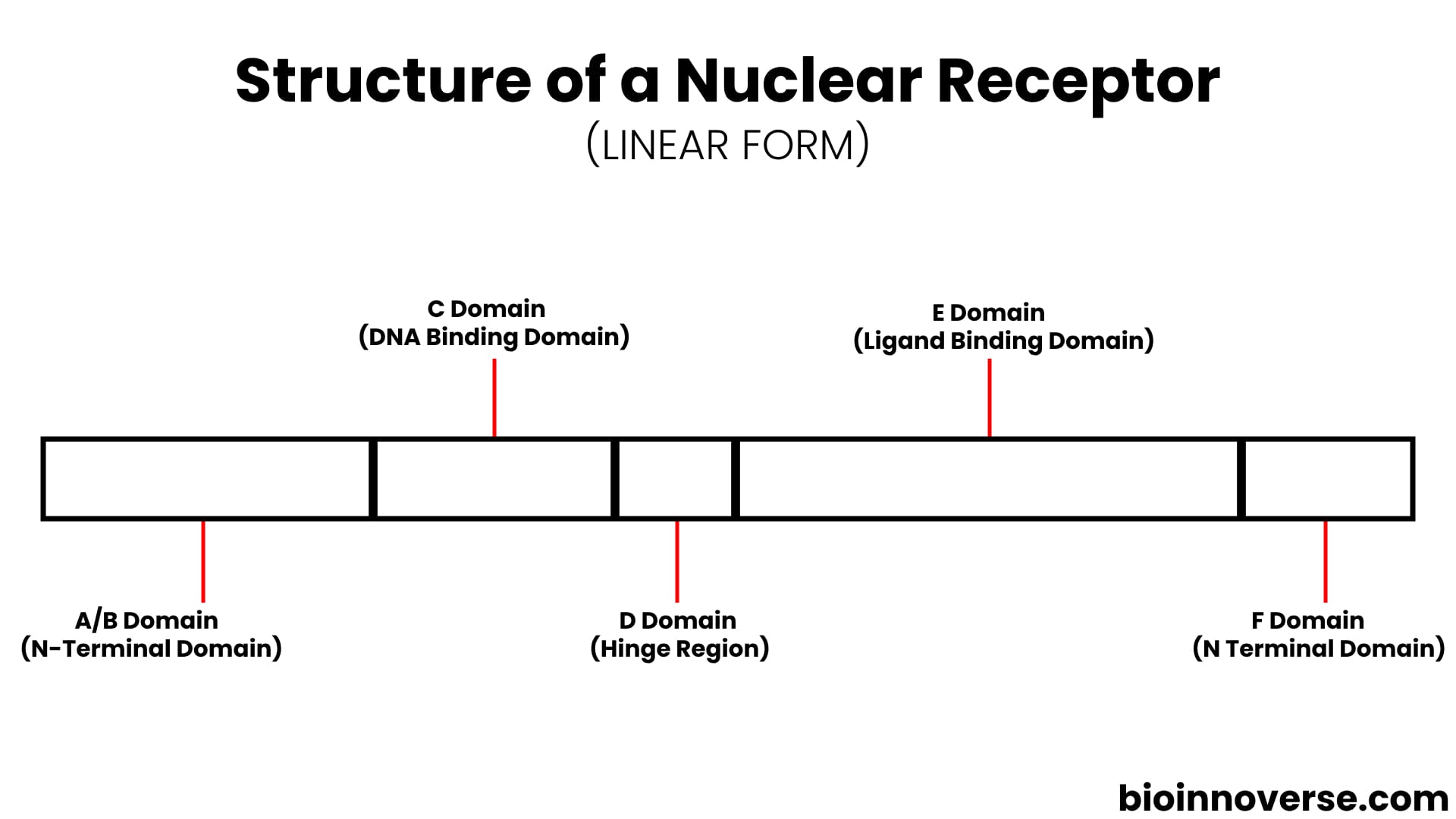
A/B Domain (N-Terminal Domain)
The A/B domain, also known as the N-terminal domain, varies significantly in sequence and length among different receptors. It includes Activation Function 1 (AF-1), which functions regardless of ligand binding and is relatively weak in transcriptional activation on its own. However, its effectiveness improves when it collaborates with AF-2, located on the E-domain, resulting in more efficient gene expression and protein synthesis.
C Domain (DNA Binding Domain)
The C-domain, also referred to as the DNA Binding Domain (DBD), is the segment specifically designed for direct interaction with DNA. This is the most important and highly conserved domain in comparison between different receptors. Within the DBD, there are two finger-like projections known as Zinc finger motifs, where zinc ions coordinate with four Cysteine or Histidine residues in a specific arrangement, are present. These Zinc finger motifs play a pivotal role in recognizing and binding to the DNA’s specific base pair sequence, thereby stabilizing the interaction between DNA and the receptor. The specific DNA region to which it binds is known as the Hormone Response Element (HRE).
Additionally, Within the DBD some sequences contribute to the homo- or heterodimerization of nuclear receptors.
D Domain (Hinge Region)
The third domain (D Domain) is known as the Hinge Region. This is the flexible Region that links the DNA Binding Domain (DBD) to the Ligand Binding Domain (LBD), facilitating signal transmission between these domains. Due to its flexibility, this region serves a vital function in receptor curvature and movement. When a ligand binds to the Ligand Binding Domain (LBD), the LBD undergoes a structural change, which is then relayed to the hinge region, causing it to also undergo conformational changes—possibly becoming rigid or folding. This alteration exposes the Nuclear Localization Signal (NLS), a short amino acid sequence typically found in the hinge region of nuclear receptors. The exposed NLS interacts with importin proteins, leading to the transport of the nuclear receptor into the nucleus. So generally, the NLS directs receptors to the nucleus, although its location can vary among different nuclear receptors. For instance, in Androgen receptors (AR) and Retinoid X Receptor (RXR), the NLS resides in the DNA Binding Domain, but in the Estrogen Receptor (ER), it is located in the Ligand Binding Domain.
E Domain (Ligand Binding Domain)
E domain is identified as the Ligand Binding Domain and this is the place where the ligand can bind on a nuclear receptor. This ligand-binding domain (LBD) is a protein domain that is highly conserved among various receptors. Here is a simple analogy that helps you understand the structure of the LBD: Imagine the LBD as a sandwich. The two slices of bread are made up of alpha-helices, two on one side and three on the other side and the filling is made up of three anti-parallel alpha-helices. Within the sandwich’s inner portion lies the ligand-binding cavity, just below the filling. When a ligand binds to the LBD, it induces conformational changes in the ligand-binding domain (LBD).
The LBD, together with the DNA Binding Domain (DBD), contributes to dimerization. Dimerization is the process by which two nuclear receptors come together to form a complex. This complex is essential for the activation of some nuclear receptors.
Within the LBD, there is a specialized region called Activation Function 2 (AF-2), which becomes active by interacting with coactivator proteins when a ligand is bound. Its activity is controlled by the shape of helix 12 (H12). Helix 12 (H12) is a flexible helix that controls the access of coactivator proteins to the activation function 2 (AF-2) region.
Without a ligand, H12 remains flexible and obscures the AF-2 region, hindering coactivator protein binding and gene transcription initiation. Binding of a ligand to the LBD triggers a structural change in H12 that stabilizes it and exposes the AF-2 region. This allows coactivator proteins to bind to the AF-2 domain. Coactivator proteins help to recruit other proteins to the nuclear receptor complex, which leads to the activation of gene transcription. Interestingly, H12, in its flexible state, can also interact with corepressor proteins, blocking coactivator recruitment and repressing gene transcription.
So overall, the Ligand Binding Domain (LBD) functions as a versatile module within a nuclear receptor, serving as a binding site for ligands, facilitating receptor dimerization, responsible for AF-2 activation, and engaging in coactivator/corepressor binding.
F Domain (N Terminal Domain)
The F domain is a highly variable tail at the carboxy-terminal (C-terminus) of some nuclear receptors, though it is absent in others. Positioned at the very end of the nuclear receptor’s C-terminus, it is the least conserved area within the nuclear receptor superfamily. The precise function of the F domain remains unclear, and further research is required to gain a complete understanding of its structure and its role in nuclear receptor signaling.
Now Let’s discuss Type 1 and Type 2 Nuclear receptors signaling pathways.
TYPE 1 NUCLEAR RECEPTORS SIGNALING PATHWAY
Type 1 nuclear receptors include those receptors that are initially present in the cytoplasm and when ligand binds to them, they translocate into the Nucleus for gene regulation. Let’s take the Estrogen Hormone as an example which is a well-known Type 1 NR and for a better understanding, we’ll break down the entire process into individual steps.
1. Ligand Binding and chaperone Dissociation
When the nuclear receptor (NR) is unbound to the ligand, it remains in an inactive state. In this state, it associates with chaperones, possibly Heat Shock Proteins (HSPs) like HSP-90 or HSP-70. These chaperones alter the shape of the receptor by binding to its Ligand Binding Domain (LBD). However, when a ligand such as Estrogen Hormone penetrates the cell membrane and enters the cell, it engages with its respective receptor, the Estrogen Receptor (ER). This ligand-receptor interaction prompts the dissociation of HSP from the receptor, leading to the activation of the nuclear receptor.
2. Receptor-Hormone Complex Dimerization Process
As a receptor enters an active state, the Receptor-Hormone complex undergoes dimerization. Dimerization is the process where one receptor binds to another, and this can be either homodimerization or heterodimerization, depending on the receptor.
3. Migration of Nuclear Receptor Dimer into Nucleus
When a Nuclear Receptor undergoes structural changes, a signal called Nuclear Localization Signal (NLS) gets exposed. This signal can be found in various parts of the receptor, often in the Hinge region or occasionally in the DBDs or LBDs. Once the NLS is exposed, it links up with an Importin protein, thereby facilitating the translocation of the nuclear receptor dimer into the nucleus through the nuclear pore.
4. Binding of Nuclear Receptor dimer to the DNA
When a nuclear receptor (NR) dimer enters the nucleus, the two zinc finger motifs extending from the DNA Binding Domain (DBD) of the NR recognize and bind to the specific base pair sequence on DNA. This region on the DNA is referred to as the Hormone Response Element (HRE). In our current illustration involving the Estrogen receptor, the binding site on the DNA is referred to as the Estrogen Response Element (ERE).
5. Expression of Genes and Cellular Responses
After the binding of the NR dimer with a specific segment of DNA, coactivators and other transcriptional machinery are recruited for transcription, resulting in the formation of mRNA. The transcribed mRNA then travels to the ribosome, initiating the synthesis of proteins that play a crucial role in various cellular responses. In the case of estrogen hormone, the resulting protein is involved in cell growth, division, and differentiation.
TYPE 2 NUCLEAR RECEPTORS SIGNALING PATHWAY
Type 2 Nuclear receptors differ from type 1 receptors in that they are already located within the nucleus, eliminating the need to migrate from the cytosol. Let’s take Retinoid X Receptor (RXR) as an example and for a better understanding of their signaling pathway, we’re breaking down the process into individual steps.
1. Dissociation of Corepressor upon Ligand-Receptor Binding:
Within the nucleus, the Type 2 NR sits on the DNA but it remains in an inactive state, incapable of initiating gene expression. This inactivity is attributed to the binding of NCoR and SMRT Corepressor complexes to the Nuclear Receptor in the absence of a ligand. These corepressors are often linked with histone deacetylases, which remove acetyl groups from histones near the receptor-bound region, compacting chromatin and preventing transcription initiation. This repression mechanism functions similarly to applying a brake on a machine. Upon ligand binding to the nuclear receptor’s ligand-binding domain, a conformational change occurs throughout the complex. This event triggers E3 ubiquitin ligase activity, which targets specific portions of the NCoR for ubiquitin tagging and subsequent degradation via the ubiquitin-proteasome pathway, resulting in the release of the corepressor complex.
2. Coactivator Proteins participation:
As a Nuclear Receptor (NR) becomes unbound from the Corepressor, it forms a dimer. Taking RXR as an example, it typically forms a heterodimer. Following the dissociation of the Corepressor Complex, the NR dimer binds with a Coactivator. This coactivator association recruits additional components, such as Histone Acetyl Transferase (HAT), Histone Methyl Transferase (HMT), and nucleosome remodelers, collectively forming an “Activator Complex.” HAT acetylates histones near this region, making the chromatin more accessible to transcription factors, while HMT methylates specific histone residues. Nucleosome remodelers adjust nucleosome spacing and density, creating accessible sites for transcription initiation.
3. Employing other Proteins for Transcription and Cellular Responses:
Finally, the Activator Complex facilitates the binding of General Transcription Factors (GTFs) and RNA Polymerase II to the promoter region for mRNA synthesis, leading to the formation of proteins. This resulting protein plays a crucial role in shaping cellular functions. Specifically, for RXR, the formed protein contributes to processes like differentiation and embryonic development.
